He called that number the order of the. Scientist Henry Gwynn Jefferies Mosle examined the X-ray spectrum of various elements in 1913-to 1914.

3 Ways To Calculate Atomic Mass Wikihow Teaching Chemistry Atoms And Molecules For Kids Chemistry Worksheets
1130 am - 500 pm lunch.

. This means the number of electrons and the number of protons in an element are equal. An electron is therefore considered nearly massless in comparison with a proton or a neutron and the. Sublevels are made up of orbitals with the same energy.
Thus the atomic number of Na atom number of electrons number of protons 11. It carries a negative charge of 1602176634 1019 coulomb which is considered the basic unit of electric charge. A neutral atom has the same number of protons and electrons charges cancel each other out.
Orbitals are the name for these sections. Electron lightest stable subatomic particle known. The 2p 3p 4p etc can each hold six electrons because they each have three orbitals that can hold two electrons each 326.
A chemistry educator tutor writer and author youll find my deep expertise applied wherever the need for an explanation of chemistry exists where a chemistry story needs to be told and where a void in communicating chemistry to children students or an adult audience is found. Finally subtract the charge from the atomic number if. You can find the number of neutrons if you know the isotope of the atom.
The 26th element in the periodic table is iron. If the number of electrons is different from the nucleuss electrical charge such an atom is called an ion. Atomic Number Orbital Energy Levels.
When an electron is at a specific energy level it is more likely to be found in certain portions of that level than others. An ion has an unequal number of protons and electrons. How to easily find the number of electrons protons and neutrons in an oxygen atom.
500 pm - 930 pm dinner. The 3d 4d etc can each hold ten electrons because they each have five orbitals and each orbital can hold two electrons 5210. The element of group-8 is iron and its symbol is Fe.
Each orbital has its own set of quantum numbers such as energy angular momentum and projection of angular momentum and only a discrete set of these orbitals exist around the. The results of his experiments show that each element has a unique integer equal to the number of positive charges in the nucleus of that element. The energy from the photon can be transferred to an electron exciting the electron out of the valence band and forming an electron-hole pair.
Therefore the number of electrons in oxygen is 8. One of the easiest ways to find valence electrons is by checking out the elements place in the periodic table. Build an Atom - PhET.
To find the number of electrons an atom has start by looking up the element youre working with on the periodic table and locating its atomic number which will be in the upper left-hand corner of the square. Iron is a transition element. If the charge is negative electrons are in excess.
500 pm - 1000 pm dinner. The services that I offer. The easiest way to find the atomic mass of an element is to look on the periodic table.
A maximum of two electrons can be. Therefore the valence electrons of iron are determined differently. Moreover since these two subatomic particles electrons and protons have opposite charges they cancel out and.
If there isnt any number or signs then it means that atom has no charge and is neutral. The number of electrons in an atom is equal to the atomic number of an element for neutrally charged species. This is due to electrons having a really small mass therefore not significantly contributing to the atomic mass.
The atomic mass can also be calculated by adding the number of neutrons and protons. If the charge is positive there are more protons than electrons. Hot electrons can be created when a high-energy photon of electromagnetic radiation such as light strikes a semiconductor.
Therefore an element in a neutral state will have the same number of protons and electrons. The wave-like behavior of a bound electron is described by a function called an atomic orbital. The valence electrons of an element can be found by closely examining the vertical column in which the elements are grouped.
For example boron B has an atomic number of 5 therefore it has 5 protons and 5. Determination of Valence Electrons. Thus to find the number of electrons possible per shell.
Then identify the charge of the ion which will be written as a superscript to the right of the element. Lastly the charge is on the upper right corner. 1130 am - 500 pm lunch.
How to find the Atomic Mass. The rest mass of the electron is 91093837015 1031 kg which is only 11836the mass of a proton. This article discusses in detail how to easily calculate the number of valence electrons in iron.
The elements which are in the same groups have the same number of electrons in outermost orbit ie 1st group has 1 electron in outermost orbit 2nd has 2 electrons in outermost orbit 13th group has 3 14th has 4 and so on. Rules to Finding Number of Protons Neutrons and Electrons of protons atomic number of neutrons mass number atomic number of electrons atomic number charge. Determine the number of electrons.
An oxygen atom. If the electron receives enough energy to leave the valence band and to surpass the conduction band it. Protons are particles in the nucleus of an atom that have a positive charge equal to 1.
Electrons are particles that have a negative charge equal to -1. For example hydrogen has just 1 electron in the s orbital of the first shell so its electron configuration notation is recorded as 1s1. By looking at the group number that is given we can identify the number.
For atoms the standard notation consists of a series of atomic subshell labels for example phosphorus sequence of notation is 1s 2s 2p 3s 3p where the number of electrons assigned to each subshell is used as a superscript. FlexBook Platform FlexBook FlexLet and FlexCard are registered trademarks of CK-12 Foundation.

How To Find The Number Of Protons Neutrons And Electrons From The Periodic Table Youtube Neutrons Proton Neutron Electron Protons

Finding Protons Neutrons And Electrons Through The Atomic Number And Neutrons By Mass Atomic
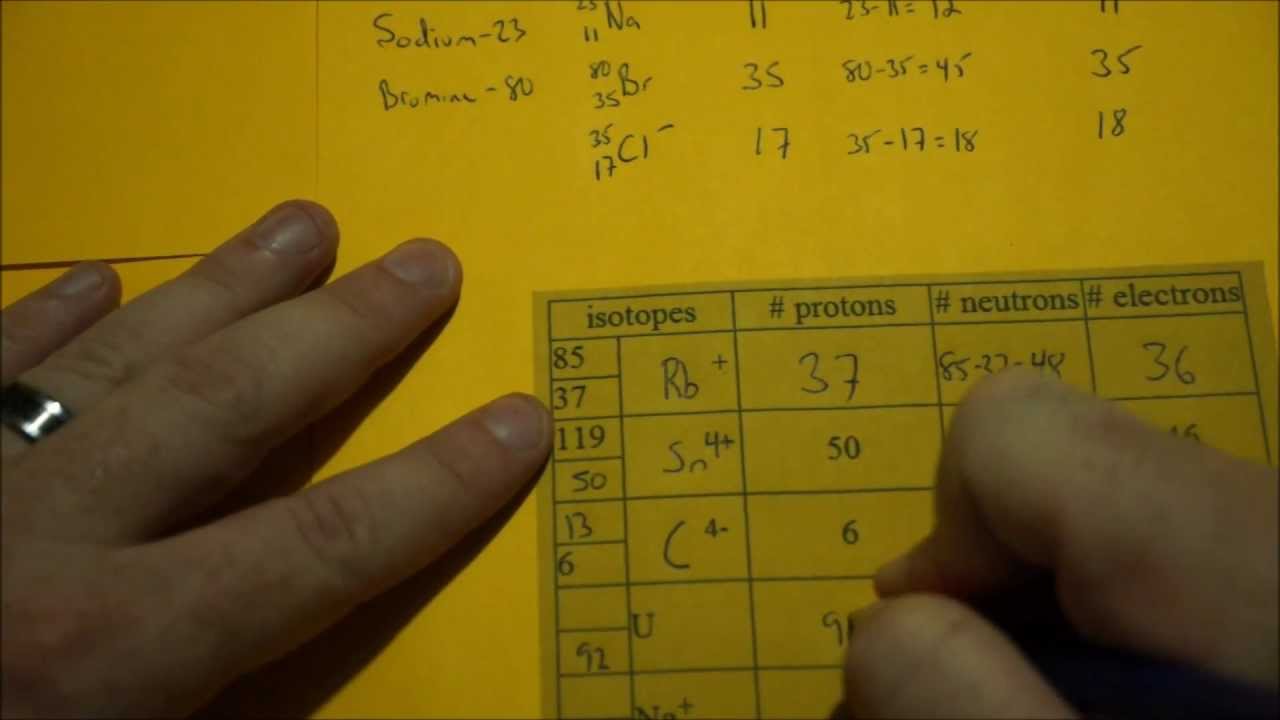
Determining Protons Neutrons And Electrons Atoms And Ions Protons Neutrons Electrons
0 Comments